A man walks past the entrance of Uganda Virus Research Institute (UVRI) headquarters in Entebbe last month. Scientists at UVRI say they are halting the HIV vaccine study. PHOTO/FILE
The chief investigator in the recently halted HIV prevention vaccine study has said HIV test results of the trial vaccine recipients are coming back positive even when they don’t have HIV.
Prof Pontiano Kaleebu, the Uganda Virus Research Institute (UVRI) director and the chief investigator in the study named PrEPVacc, said: “We need to follow them up because if you test them, they are positive. And that is what we call vaccine-induced seropositivity.”
“But it doesn’t mean they have the virus, no, it’s the antibody they have because the tests just look at the antibodies and find them there but their bodies were induced by the vaccine to produce those antibodies. And we need to counsel them until that seropositivity goes away,” he explained.
Some Ugandans are often subjected to HIV tests before they are given jobs and allowed into some countries to work or start the marriage process.
Although the Uganda Aids Commission says it is illegal to subject someone to an HIV test as a requirement for getting or maintaining a job, the vice has persisted, with those who test positive barred from the opportunities.
According to a March 2024 analysis report by Will Colón of the Johnson & Johnson Global Public Health Research and Development in Belgium and colleagues which was published in the Journal of Infectious Diseases, a scientific journal, the inability of the existing tests to distinguish between the vaccine-induced positivity and positivity from real HIV infection is a threat to HIV vaccine studies and uptake.
“Current serologic tests for HIV screening and confirmation of infection present challenges to the adoption of HIV vaccines. The detection of vaccine-induced HIV-1 antibodies in the absence of HIV-1 infection, referred to as vaccine-induced seropositivity/seroreactivity, confounds the interpretation of test results, causing misclassification of HIV-1 status with potential affiliated stigmatisation,” the report reads.
“For HIV vaccines to be widely adopted with high community confidence and uptake, tests are needed that are agnostic to the vaccination status of tested individuals (that is to say, positive only for true HIV-1 infection),” they added.
Desired outcome
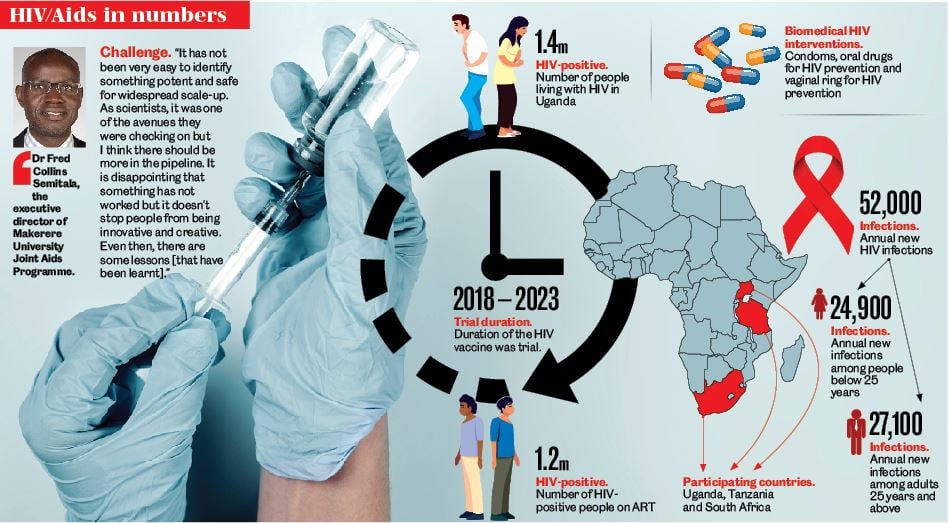
The halting of the vaccinations among 1,500 volunteers in Uganda, Tanzania and South Africa, was announced last year in December after it was discovered that the HIV prevention trial vaccine was not yielding the desired outcome –prevention of HIV acquisition among study participants.
According to the researchers, the study enrolled healthy, HIV-negative individuals to participate in the trials. The participants were from the general and key populations who reported behaviours that put them at increased risk of catching HIV. This included female bar workers, people living and working around main highways, commercial sex workers, fisherfolk and men who have sex with men. The researchers started enrolling people in 2018 and the volunteers were told what to expect, the safety and issue of VISP (vaccine-induced seropositivity) and they were allowed to leave the study anytime.

Prof Pontiano Kaleebu, the UVRI director and the chief investigator. PHOTO/TONNY ABET
“The good thing is that the vaccine was safe. There were no safety concerns. It was only stopped because the International Data and Monitoring Committee told us that even if we continued, there was no way the vaccine was going to work. So they told us to stop vaccinations. But the volunteers are safe,” Prof Kaleebu said.
According to information from the PrEPVacc website, “It is not possible to get HIV infections from the vaccines used in this study. The vaccines do not contain any live HIV, killed HIV, or parts taken from HIV or HIV-infected cells. The vaccines are made up of safe chemical components.”
Information from the PrEPVacc also indicates that the study involved two combination regimens. The two regimens trialled in PrEPVacc included “one combining Deoxyribonucleic Acid (DNA) with protein-based vaccine, and the other combining DNA, Modified Vaccinia virus Ankara (MVA) and protein-based vaccine,” according to the researchers.
The researchers also indicated that regimens had already been evaluated in multiple phase I/II clinical trials in the United States, Europe and Africa and have demonstrated their safety and ability to induce immune responses. The providers and managers of vaccine regimens include the US-based Global Solutions for Infectious Diseases, The Netherlands-based EuroVacc Foundation, and Imperial College London.
PrEPVacc researchers also indicated that during the study, participants were counselled on HIV prevention methods but they were allowed to go on with their normal life, meaning they could still get infected when they engage in unprotected sex with an HIV-infected person.
According to information from the Uganda Aids Commission, more than 50,000 Ugandans contract HIV every year, with around 17,000 HIV-related deaths among the estimated 1.4 million Ugandans living with HIV. Scientists are looking for an HIV prevention vaccine to reduce infections to attain the goal of ending HIV as a public health threat by 2030.
The chief investigator explained that during the study, in addition to the vaccine, they gave the volunteers Pre-Exposure Prophylaxis (PrEP)-an infection-prevention drug.
“We gave PrEP to all these individuals. What we do in these trials now is to provide all prevention measures that exist,” Prof Kaleebu said.
The UVRI director said they are following the recipients up to understand the behaviour of the virus in recipients who got infected.
“Those are some of the studies we are going to do. To look at these viruses in individuals who are vaccinated, when they got infected, what happens to the virus? We hope it will not be more virulent,” he added.
He said this during the meeting with members of the Health Journalists Network in Uganda (HEJNU) on Thursday in Kampala where issues around vaccine hesitancy were discussed.
Taking 20 years, way out
According to information from the HIV Vaccine Trials Network (HVTN), an HIV-negative vaccine recipient can continue to test positive for up to 20 years when they are using standard tests that look for antibodies.
“If you have tested Vaccine-Induced Seropositive (VISP), the antibodies may fade quickly or they may last for several years. In some cases, participants continue to test VISP for more than 20 years,” information from HVTN reads further.
“If someone believes you have HIV, you could face discrimination and/or other problems. For example, you could have problems with medical or dental care, employment, insurance, a visa for travelling, or entry into the military. You might not be allowed to donate blood or other organs. If you are pregnant, you may have to explain your situation to avoid receiving any HIV treatment during your pregnancy or labour/delivery,” the organisation adds.
However, the HVTN states that specific tests that look for the virus (Polymerase Chain Reaction –PCR) can distinguish between vaccine-induced seropositivity and a positive test resulting from a real HIV infection.
“Standard HIV tests that look for antibodies are quick, reliable and affordable. Tests that look for the virus (PCR) are expensive and not commonly used for an initial diagnosis,” information from HVTN reads.
In Uganda, a PCR test can go for over Shs150,000, an amount which is too high when compared to a common HIV test (detecting antibodies) that may cost Shs15,000 or less. Some of the kits are also given for free by the government.
New trials by Makerere
The halting of the PrEPVacc study and issues around VISP could be causing headaches to scientists and health professionals but this has not quenched the fire in the HIV vaccine search.
Makerere University Walter Reed Project (MUWRP) is collaborating with American scientists to test another HIV vaccine in Uganda, according to information from the US Embassy in Kampala and the university.
The management of the MUWRP told this publication: “Currently, MUWRP is collaborating with the US Military Health Research Program (MHRP) to conduct a novel HIV vaccine study named “RapidVax”.”
They continued: “The screening and enrolment of participants commenced in March 2024 and are expected to end in July 2024. To date, 46 participants have been screened and eight participants enrolled. The study intends to enroll 50 participants in total.”
“The strategy for this particular study mimics the early-stage HIV viral dynamics by administering vaccines more frequently than traditional ways of vaccine administration. The researchers leading this study hypothesise that RapidVax repeated immune system stimulation can enhance the body’s ability to defend itself against infections,” they added.